Science at Home! An Eggs-celent Adventure
For immediate release ‐ April 16, 2020
Contact: Jessica Wackes, 919.707.9850. Images available upon request
Are you looking for something to do to keep your brain active and engaged? We’re here to help with Science at Home! Over the next few weeks, we’ll be providing you with fun science experiments you can conduct using commonly found items. You can also visit us at the Museum’s Science at Home page for additional resources!
Find a printable version of Experiment #1 here, and a printable version of Experiment #2 here.
Experiment #1
Materials Needed:
2 squares of cardboard or foam board (roughly 1 foot square)
3 raw chicken eggs
A pile of books
Optional: bathroom weight scale

Experiment Instructions:
Step 1: Place one cardboard square on a table and using your knuckle make three dents equally spaced in an equilateral triangle. Six inches apart is a good distance, but can be less.
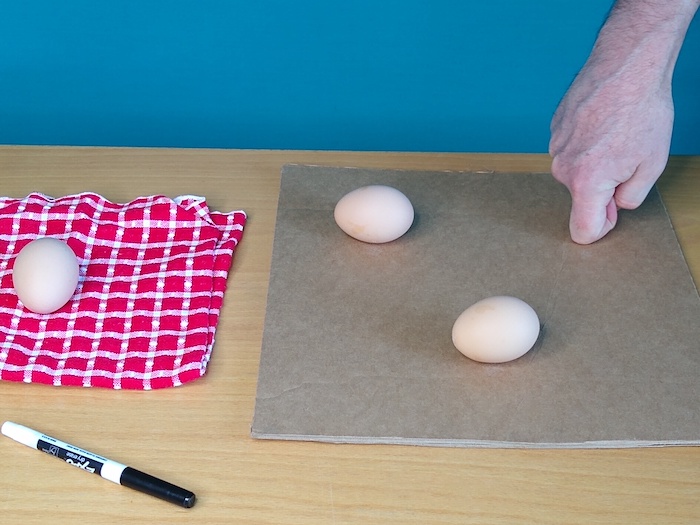
Step 2: Place one egg in each dent. (The dents keep the eggs from rolling away.) Now place the second square of cardboard on top.
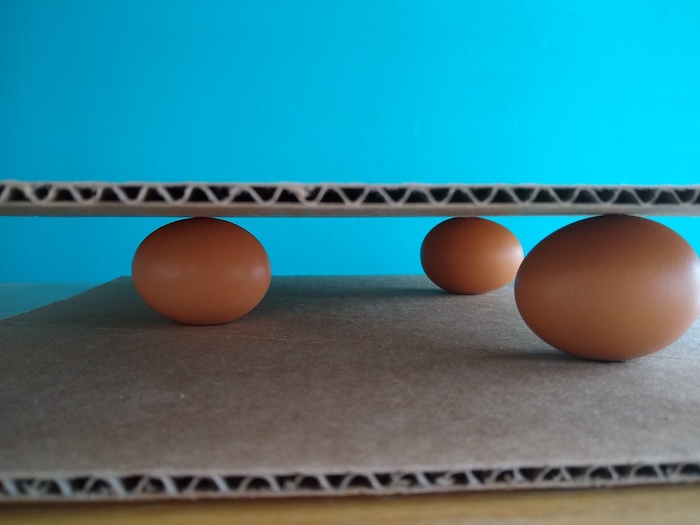
Step 3: Now carefully begin to stack books on top of the cardboard; start with your largest, widest and heaviest books. Stack slowly and carefully and see just how many books you can stack before the eggs crack. You will be very impressed how tall your stack becomes before the eggs crack!

Optional Step: If you have a bathroom scale, you can weigh the books (minus the final one that brought upon the crash!) and see how many pounds these three eggs supported. You’re likely to find the results surprising!

What We’ve Learned
Eggs are naturally curved. Almost anything with a curve in its design is strengthened by this simple structural addition.
Think of Roman arches in 2000-year-old aqueducts or Inuit igloos made only from snow. Curvature imparts surprising strength.
Experiment #2
Materials Needed:
1 raw chicken egg
1 coffee mug (or similar-sized container)
Household vinegar

Experiment Instructions:
Step 1: Place egg in mug and fill with vinegar until egg is completely submerged. Let sit for at least 24 hours.

Step 2: After 24 hours or more, remove egg from vinegar and gently rinse under the faucet using your fingers to help scrub away any remaining residue.
Note: Be gentle as this egg no longer has a shell to protect it.

Step 3: You now have a “rubber” egg!
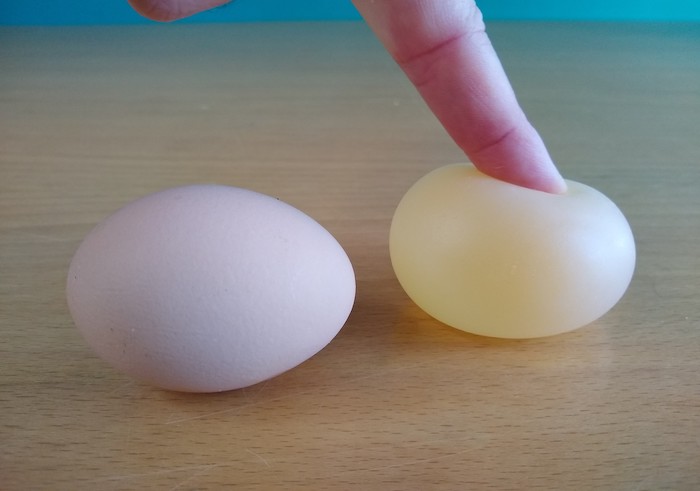
What We’ve Learned
This “rubber” egg is exactly what it looked like inside the hen before calcium carbonate was applied to create the hard shell.
During egg laying, hens develop a special bone tissue called medullary bone which enables calcium in the chicken’s bones to make its way to the egg. The finished shell is composed mostly of calcium carbonate, which dissolves easily in acid. (Vinegar is weak acid.)
Carbon dioxide is a by-product of the chemical reaction taking place and is seen as numerous bubbles that appear immediately after placing the egg in the vinegar! After about 24 hours, the shell has dissolved leaving only the interior membrane behind.
Having Fun?
We want to see! Tag @naturalsciences on social media so we can see you and your loved ones enjoying our Science at Home experiments.
Want More Experiments?
Try this one about Water Density!
Try this one about Water Tension!
For more information about our upcoming activities, conservation news and ground-breaking research, follow @NaturalSciences on Instagram, Twitter and Facebook. Join the conversation with #visitNCMNS.

